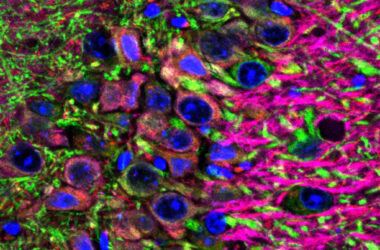
The collaboration between the «Cell Division Control » research group led by Professor Juan Jiménez and the «Architecture and nuclear dynamics» group led by Professor Rafael R. Daga, both researchers at the CABD – a mixed research centre participated by the Pablo de Olavide University, CSIC and the Regional Government of Andalusia – has led to the discovery of an adaptive mechanism that explains why cells break down their nuclear envelop each division cycle. According to their study, recently published in Cell Reports -one of the most prestigious journals on Cell Biology-, ending cell division in fission yeast requires disassembly of the mitotic spindle -the structure of microtubules specialized in chromosome segregation- at the end of mitosis by action of disassembly factors which localize in the cytoplasm. Therefore, opening the nuclear envelop may has an evolutionary origin.
Many properties of human cells at present may be better understood from a historical perspective, going back to their evolutionary origin. Early eukaryotic cells probably emerged some 2.2 billion years ago by creating an internal membrane structure – nucleus – that separated their genetic information – DNA into chromosomes – from the other components and processes of the cell. In these early simple and small eukaryotic cells, it can be expected that the nucleus would remain intact throughout the life cycle, including the time when the genetic information is duplicated and segregated into two daughter cells – mitosis. At present, simple eukaryots whose cells maintain the nuclear envelop during mitosis such as yeasts and fungi are still found in nature. But for some reason, in the eukaryotic cells of today’s more complex organisms, from nematodes or insects to our own cells, the nuclear envelop breaks down every time the cell enters into mitosis, a striking process with an enormous cost of energy and exposure of the genetic material.

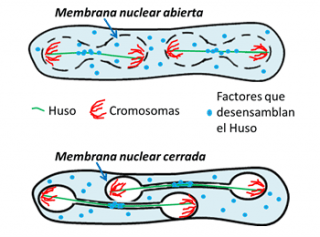
Yeast cells always keep the nucleus closed, therefore, these cells have to transport spindle disassembly factors through the nuclear envelop at the end of mitosis, as likely occurred in early eukaryotic cells. Interestingly, in a special division – the second meiotic division – that yeast makes to produce its gametes – spores – the yeast cell partially breaks down the nuclear membrane barrier, something that in practice is similar to what happens in our own cells, breaking down the nuclear membrane. According to Juan Jiménez, «this work reveals that breaking down the nuclear barrier in these yeasts allows the spindle disassembly factors to access the nucleus earlier, without the need of nuclear membrane transport. This ‘short-circuit’ causes the spindle to disassembly earlier, thus allowing it to be much shorter and the chromosomes to secrete in a more limited space in the cell during the second meiotic division».
This function in shortening spindle length by opening the nuclear membrane barrier in yeast leads these researchers to extrapolate a possible similar utility for opening the nuclear envelop during eukaryotic evolution: when early cells increased in complexity and genetic information, the length of the mitotic spindle does not fit into the limited volume of the nucleus after a critical cell size. Thus, the rupture of the nuclear envelop may have arisen as an adaptive mechanism to reduce the spindle length, allowing it to proceed without interference in a limited cell volume during mitosis. The study has been selected by the magazine as «featured article», accompanied by a preview describing its relevance.
Cell Reports: Ignacio Flor-Parra, Ana Belén Iglesias-Romero, Silvia Salas-Pino, Rafael Lucena, Juan Jimenez and Rafael R. Daga. Importin α and vNEBD control meiotic spindle disassembly in fission yeast. Cell Reports, 23:933-941. 2018. DOI: https://doi.org/10.1016/j.celrep.2018.03.073
http://www.cell.com/cell-reports/fulltext/S2211-1247(18)30434-0